What Word Describes the Mathematical Relationship Between Mass and Volume
V 2 new volume. You must wait 6 hours between the two sets.
Density Formula How To Calculate Density
With phase changes states of matter due to the particle arrangements in solids liquids and gases.

. Physicists calculate the weight of an object by multiplying its mass by the gravitational force acting on it using the formula Wmg where W is the weight M is the mass. VOLUME GRAPHS On these graphs the axes represent the following. P 1 old pressure.
In a closed gas-filled container individual molecules of the gas are constantly bouncing. Y mx b or time m volume b a constant 2 Quadratic. VOLUME GRAPHS Slope of a line.
To represent this scenario in mathematics a scientist named Robert Boyle derived the mathematical relationship between Pressure kPa and Volume L in 1662 where Pressure kPa x Volume L a Constant K PV K. The mass of a substance per unit of volume. Inversely porportional The relationship between density and volume.
Y C x2 where C is a constant and x is volume. Boyles law describes the relationship between pressure and volume at a constant temperature for a fixed mass ie number of molecules of a gas. For a given mass and volume how much physical space a material takes up of an object or substance the density remains constant at a given temperature and pressure.
Mass is the amount of matter an object contains while volume is how much space it takes up. The graph of this equation is shown below. P 2 new pressure.
Prelaboratory Assignment The pre-lab assignment for this experiment is a TRQ on significant digits. Y axis ² represents MASS on MASS VS. As the gravitational force exerted by Earth is the constant upon which mass is calculated the values of an objects mass and weight on Earth are approximately equal depending on geographic location.
Customary system uses feet quarts and ounces to measure these. Since P times x V is equal to a constant. K then it follows that different conditions of pressure and volume for the same mass of gas at constant temperature can be expressed as.
To find mass use the triple beam balance. We can write a mathematical relationship to show this ratio as follows. Then decide the form of the equation.
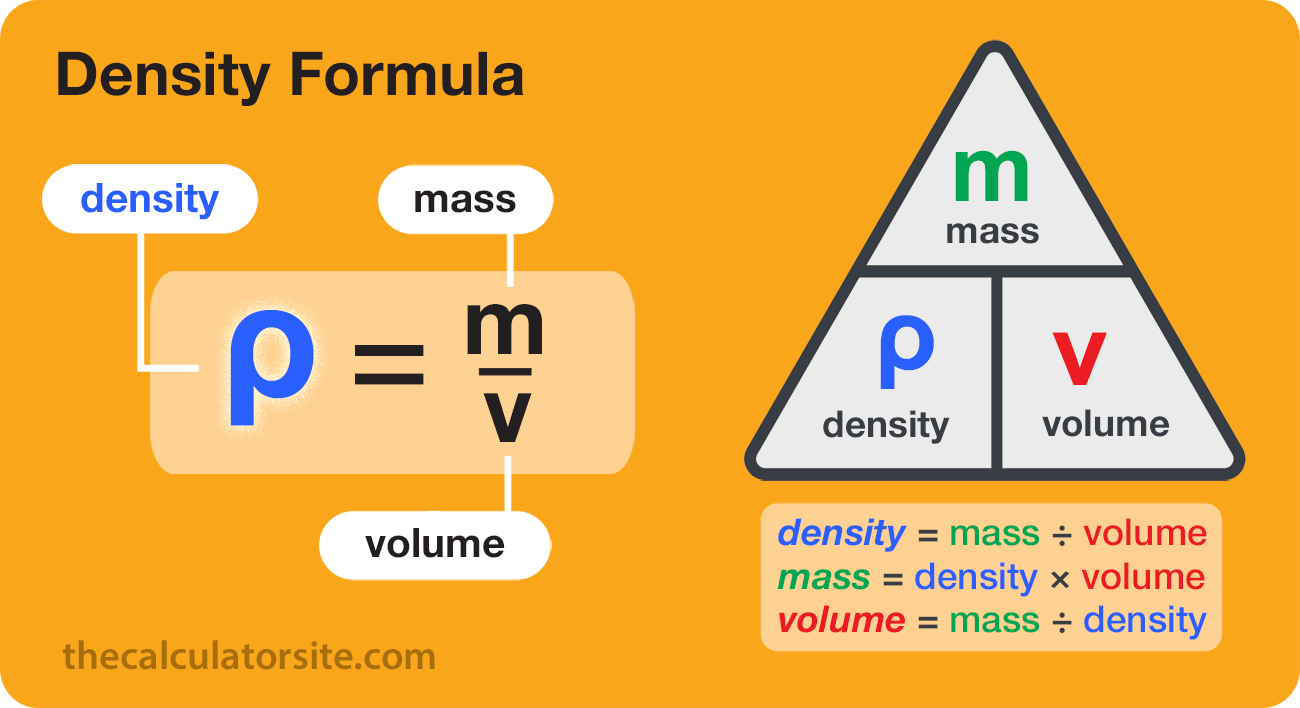
Density is defined as mass per unit of volume. VOLUME GRAPHS X axis - represents VOLUME on MASS VS. The equation for this relationship is ρ m V in which ρ rho is density m is mass and V is volume making the density unit kgm3.
Start with the volume in Column A and the time in Column B. We say density is the ratio of mass to volume. Where G is the universal gravitation constant 667 X 10-11 Nm2kg2 m1 is the mass of the first object in kilograms m2 is the mass of the second object in kilograms and r is the distance between the centers of the two masses in meters.
Heres how to fit an equation to your data using a spreadsheet. Refers to the VWHHSQHVVµ o f a line VLPLODUWRWKHVWHHSQHVVµRID hillmountain Slope of a line ² shows rate of change ². To be precise when using measurement terms make sure you know the difference between mass and weight.
A logical corollary to Avogadros hypothesis sometimes called Avogadros law describes the relationship between the volume and the amount of a gas. DO NOT BEGIN YOUR EXPERIMENT UNTIL EACH MEMBER OF YOUR GROUP HAS READ THE BACKGROUND AND ANSWERED THE BACKGROUND QUESTIONS. In addition to the difference in the basic units the metric system is based on 10s and different measures for length include kilometer meter decimeter centimeter and millimeter.
Boyles Law sometimes referred to as the Boyle-Mariotte Law states that the absolute pressure and volume of a given mass of confined gas are inversely proportional provided the temperature remains unchanged within a closed system. V 1 old volume. P 1 V 1 K P 2 V 2 thus P1V1 P2V2 equation 1 where.
The units for density are gmcm3. Density is calculated using the formula. The graphical relationship will be used to predict the volume of an unknown sample based on its mass.
What determines the concentration of a solution. Learn about the relationships between moles liters and molarity by adjusting the amount of solute and solution volume. For a given mass and volume how much physical space a material takes up of an object or substance the density remains constant at a given temperature and pressure.
The equation for density is DMV. The equation for this relationship is. Density is a measure of how much mass of a material fits into a given volume.
Rho fracmV in which ρ rho is density m is mass and V is volume making the density unit kgm3. As the mass of an object increases or decreases the density of the object increases or decreases. A bowling ball and a basketball are about the same volume as each other but the bowling ball has much more mass.
At constant temperature and pressure the volume of a sample of gas is directly proportional to the number of moles of gas in the sample. To understand Boyles law it helps to visualize the behavior of gas molecules in an enclosed space. Density of a substance changes.
The metric system uses units such as meter liter and gram to measure length liquid volume and mass just as the US. Change solutes to compare different chemical compounds in water. Number Mass m Initial Volume V i Final Volume V f and Slug Volume V slug.
You must complete the quiz TWICE in order to receive full credit.

Y Mx B Key Words For Word Problems Teaching Algebra Learning Math Secondary Math

Density Volume Mass Flashcards Quizlet

Density Lesson For Kids Definition Facts Video Lesson Transcript Study Com
No comments for "What Word Describes the Mathematical Relationship Between Mass and Volume"
Post a Comment